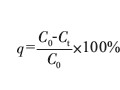摘 要:The electrochemical activity and stability of tungsten carbide gas diffusion electrode in different electrolytes were determined by galvanostatic charge method. It is shown that WC exhibits good electrocatalytic activity and stability for hydrogen oxidation in acidic solutions when the electrode potential is below about 800 mV (vs DHE), WC is firstly oxidized to an unstable blue tungsten oxides at 800-900mV which are closed to a composite stoichiometry of W2O5 in H2 SO4 solution and W8O23 in HCl solution calculated by charge consumed. Furthermore,the generated intermediate tungsten oxides can be further oxidized into WO3 at higher potentials. While in alkali solution, WC can not be used as anodic catalyst for its poor stability and catalytic activity due to the fact that WC will be directly oxidized into WO3.
![]() Electro-oxidation behavior of tungsten carbide electrode in different electrolytes.pdf
Electro-oxidation behavior of tungsten carbide electrode in different electrolytes.pdf